In a recent study published on the medRxiv* preprint server, researchers describe the kinetics of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-specific serum antibodies in convalescent children over six months following infection.
Study: Dynamics of infection-elicited SARS-CoV-2 antibodies in children over time. Image Credit: Maria Sbytova / Shutterstock.com
SARS-CoV-2 infection induces an immune response that targets many viral proteins, including the spike (S) and nucleocapsid (N) proteins. The S protein is a major target of neutralizing antibodies, as it binds to the host angiotensin-converting enzyme 2 (ACE2) receptor during viral entry.
Although previous studies have shown that coronavirus disease 2019 (COVID-19) in children often causes milder symptoms as compared to adults, data on long-term circulating antibodies to SARS-CoV-2 in the pediatric population is sparse.
About the study
In the present study, researchers assessed the anti-N binding antibody levels and anti-S neutralizing antibody titers in unvaccinated children. These responses were then compared to titers of unvaccinated older adults following SARS-CoV-2 infection.
The researchers analyzed a group of SARS-CoV-2-infected convalescent children under 18 years of age for up to 52 weeks after symptom onset. They measured levels of anti-S neutralizing antibodies using a pseudoneutralization assay and compared the pediatric immune response to the antibody response of a previously characterized adult cohort. The adult cohort also included older adults, most of whom experienced mild SARS-CoV-2 infections and did not require hospitalization.
Children aged under the age of 18 years, including children with comorbidities, were enrolled in the study. Sera for the immune response assessment was obtained from the Seattle Children’s Hospital, Seattle starting in April 2020.
The researchers used the REDCap electronic data collection tool to obtain data on patient demographics, hospitalization, clinical information such as respiratory support, length of stay, intensive care unit admission (ICU) admission, and medical history, including underlying conditions. This tool also provided lab reports, including testing results.
Children with presumed or confirmed SARS-CoV-2 infection were included in the study between April 2020 and January 2021. While reverse-transcriptase polymerase chain reaction (RT-PCR) positivity was a requirement for confirmed SARS-CoV-2 infection, children with no positive RT-PCR documentation were presumed to be infected by SARS-CoV-2 if they had detectable antibodies specific to SARS-CoV-2.
Study findings
Between April 2020 and June 2021, a total of 97 pediatric patients were enrolled in the study, with 42 of them being followed up for at least six months following infection. Thirty-two of these 42 children had presumed or confirmed infection.
In the 25 children who did not have immunocompromising conditions or multiple blood transfusions, very little variation was observed in overall neutralization titers over the 24-week period. A total of 15 out of the 25 children had a reduction of at least 25% in their neutralization titers over the course of the 24 weeks. All immunocompetent children showed neutralizing activity at all time points.
The observations showed lower neutralizing antibody titers in children as compared to adults in the early stages of SARS-CoV-2 infection. However, titers were similar across different age groups six months following infection.
Levels of anti-N antibodies dropped significantly in children, with 18 of the 23 children positive for anti-N antibodies showing a more than two-fold decrease and the remaining five showing a less than two-fold decrease. An increase in anti-N antibodies was not observed in any of the children in the study. Overall, children without underlying conditions exhibited similar trends in the reduction of anti-N antibody levels over time.
The neutralizing activity of sera from children slightly decreased from one to six months after infection, which was not significantly different from the activity observed in adults. However, SARS-CoV-2 infection of children elicited much lower amounts of anti-N antibodies that also declined more rapidly when compared to adults and older adults. Taken together, these results underscore age-related differences in antibody responses against SARS-CoV-2 S and N proteins.
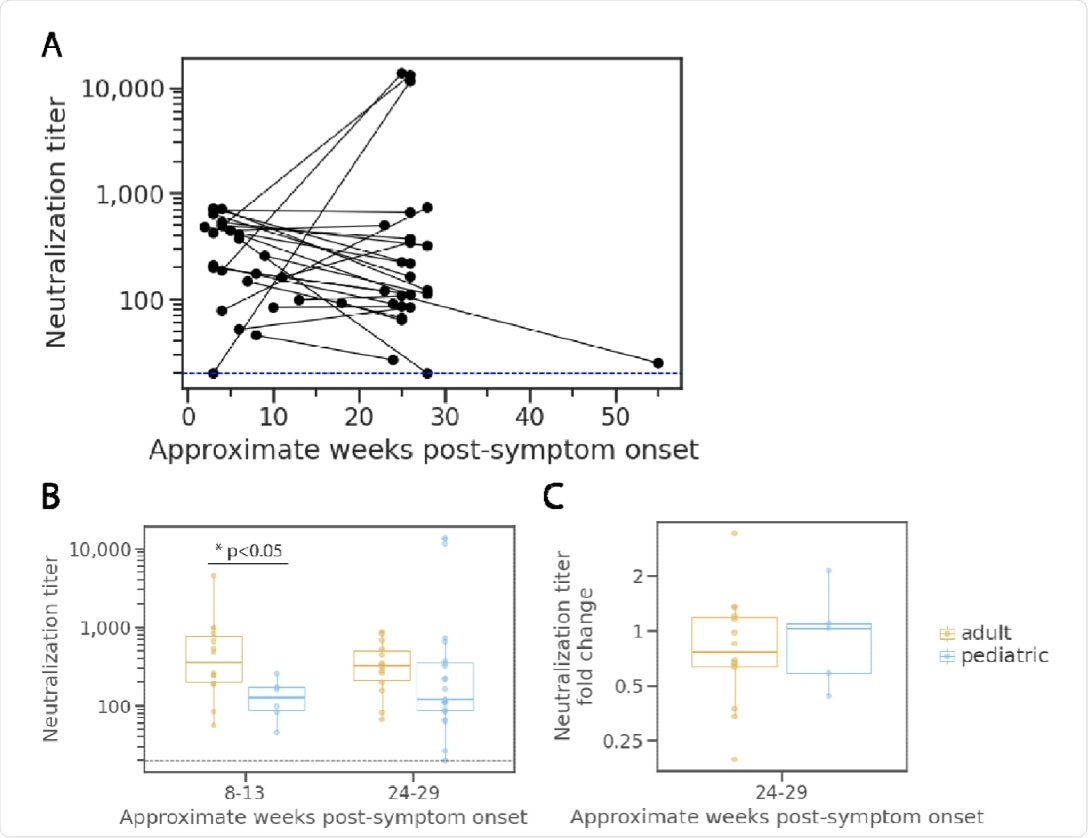
Neutralization potency kinetics in children compared to adults.
Conclusions
Antibody responses to SARS-CoV-2 can vary in children and adults because of the lower disease severity and immune senescence in children, as well as a relatively increased burden of comorbidities in adults and older adults. However, the results of this study did not show significant age-related variations in SARS-CoV-2-specific antibody responses between children and adults.
Interestingly, the observations showed only a modest and non-significant decline in the levels of neutralizing antibodies in pediatric sera collected over six months following infection. A similar result in the adult cohort suggests that the long-term maintenance of neutralizing antibodies following SARS-CoV-2 infection is independent of age.
The most prominent difference observed in levels of SARS-CoV-2 antibodies between children and adults was for anti-N antibodies. Children had lower anti-N antibody levels as compared to adults both, during the early stages of infection and six months following infection; however, this difference was not statistically significant.
Taken together, the findings from the current study offer valuable insights into the immune responses to SARS-CoV-2 in children over time. Furthermore, the researchers here provide long-term data on SARS-CoV-2 neutralizing antibodies that are crucial for comparison investigations of vaccine-induced immunity in children.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
