A new study published on the preprint server bioRxiv* in August 2020 shows that the man-made compound silicon nitride, which is used in medical implants and high mechanical performance engineering applications, is capable of inactivating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at varying concentrations without causing cell cytotoxicity. This finding should be investigated to develop disinfectants to clear the virus from surfaces, preventing its spread.
Viral Transmission Via Air, Fomites, and Surfaces
Viruses are known to spread via fomites and contaminated surfaces. This route of transmission is of concern when these surfaces occur in crowded environments where infected individuals are likely to be present at high density. This includes schools, nursing homes, and hospitals, and daycare centers.
Sneezing, coughing, singing, and playing wind instruments have been associated with the aerosol transmission of respiratory viruses such as SARS-CoV-2. Still, the fact remains that contamination of hands and surfaces by respiratory droplets is an important reason for the continuing spread of the virus. This is attracting intense attention today as COVID-19 has achieved community spread in many hotspots around the world.
It is established that viruses are capable of surviving long-term on many surfaces. The earlier pathogenic coronavirus SARS-CoV, which was responsible for the 2002*2004 outbreak of severe acute respiratory syndrome, was found to survive for up to 9 days in suspension, and 6 days in the dried state. Another pathogenic coronavirus, HuCoV-229E, which is responsible for upper respiratory infections in humans, has been found to live for up to 5 days on a variety of surfaces, from glass to stainless steel.
The current virus, SARS-CoV-2, has also been detected live at up to 72 hours on plastic, copper, and cardboard, and for up to 7 days on surgical masks. Thus, the need for an effective surface disinfection method is pressing.
Disinfecting Surfaces
Various technologies have been used, including disinfectants, irradiation, and the use of metals like copper, zinc, iron, and silver, which rapidly inactivate viruses. Copper alloys, for instance, are used to create antimicrobial surfaces in healthcare facilities and cut down the incidence of nosocomial infections.
Disinfectants found to be effective in this situation include the quaternary ammonium compounds like ammonium chloride. These disrupt the protective lipid envelope of the virus and thus inactivate it. These compounds are therefore used to clean healthcare surfaces and remove persistent viral particles.
Silicon Nitride Spinal Implants Show Very Low Infection Rates
The current study focuses on silicon nitride, Si3N4, which is a non-oxide ceramic used under FDA approval for spinal implant surgery. Such devices have shown that the long-term outcomes are excellent when silicon nitride is used for both lumbar and cervical fusion, compared to other biomaterials used in similar situations like bone grafts, titanium, and polyetheretherketone.
In an aqueous environment, silicon nitride spinal implants are hydrolyzed from the surface, releasing ammonia at the microscopic level. This molecule is then converted into ammonium, nitrous oxide, and reactive nitrogen species, which suppress the growth and proliferation of bacteria. This is perhaps the reason for the intrinsic antibacterial properties of this compound, and why its use in spinal implants is associated with a lower incidence of bacterial infections, as low as 0.006%, in contrast to the other biomaterials that have as high an incidence of infection as ~3% to 18%.
Virucidal Activity of Si3N4
When viruses were exposed to an aqueous solution of silicon nitride, it inactivated viruses like the influenza virus H1N1, enterovirus and feline calicivirus. Earlier papers reported that silicon nitride in aqueous suspension also inactivates SARS-CoV-2 as well as copper ions. However, a significant advantage is that silicon nitride is not cytotoxic to mammalian cells, unlike copper.
The researchers in the current study tried to determine that exposure to silicon nitride would not be toxic to mammalian cells in vitro but would inactivate the virus at a rate dependent on the duration and dose of exposure.
Si3N4 Does Not Affect Cell Viability
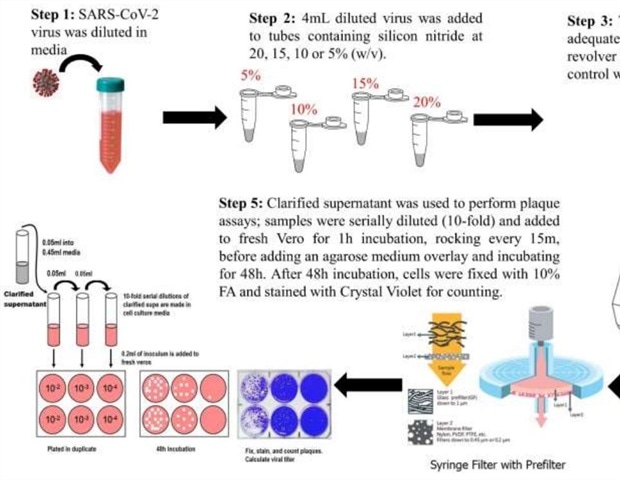
They found that when they used suspensions of silicon nitride at 5, 10, 15, and 20% (w/v), on Vero cells in culture, there was no difference in cell viability at 24 or 48 hours after exposure at any concentration except with the highest. In fact, the cell viability improved by about 10% at 48 hours after being exposed to 5% and 10% suspensions for 10 minutes. This suggests a stimulatory effect of silicon nitride on cell growth or metabolism.
At 20% w/v, the cell viability did show a small decline, of about 10%, at 48 hours after being exposed to the silicon nitride.
Si3N4 Inactivates SARS-CoV-2
The researchers also found that at the same concentrations, silicon nitride exposure caused inactivation of SARS-CoV-2, as evaluated by plaque assay. Those viruses which were exposed to cell culture media showed 4.2 x 103 PFU/mL. In contrast, all concentrations of Si3N4 reduced viral titers to varying extents. At one minute of exposure to a 5% suspension, the viral titer went down by 0.8 log10, with a 10% suspension by 1.2 log10, at 15% by 1.4 log10, and with a 20% suspension by 1.7 log10. The same trend was observed at 5 and 10 minutes.
This reduction in viral titer corresponds to 85%, 93%, 96% and 98% inhibition of viral particles at 5%, 10%, 15% and 20% suspensions, respectively. Inhibition is almost 100% when the exposure is continued for longer durations and at higher concentrations, such as 10 minutes at 20% w/v.
Thus, the researchers observe, Si3N4 has a strong inhibitory effect on SARS-CoV-2 proliferation but no cytotoxic effect on mammalian cells.
Implications
The researchers say that these findings are remarkable since a single minute of exposure to a 5% solution of silicon nitride reduces the number of active viral particles by 85%. Still, the cell is minimally affected even 48 hours after exposure at a 20% concentration of the substance. This agrees with prior research that shows that Si3N4 has a remarkable “dual effect” in that it not only produces viral inactivation but also suppresses the formation of bacterial biofilm, but spares mammalian cells.
It is also concordant with another recent study that shows the virus to be rapidly inactivated by exposure to 15% silicon nitride, as well as other data that relates this to antiviral effects of Si3N4 on a range of single-stranded RNA viruses as well, as mentioned above.
This suggests a spectrum of applications, such as developing fabrics for personal protective equipment like masks and surfaces on commonly-touched items of furniture.
Mechanism of Viral Inactivation
The researchers suggest several antiviral actions of Si3N4, such as the ability of the material to release ammonia released at a slow and controlled rate from the surface. This gives rise to reactive nitrogen species that fragment the viral RNA.
Secondly, ammonia also gives rise to free electrons and silanols with an excessive negative charge in aqueous solutions.
Thirdly, the silicon nitride surface carries protonated amino groups, Si–NH3+, which are similar to the N-terminal lysine end of the virus, C– NH3. This could lead to the competitive binding of the virus to this material and its subsequent inactivation.
Silicon nitride has several superior features over other potential antimicrobial surfaces in that it continues to offer sustained antiviral activity because of these hydrolytic reactions taking place at its surface, rather than a single disinfectant action that will require repeated and meticulous applications. Also, even though copper is known to have potent virucidal activity, it is also toxic to cells. On the other hand, Si3N4 implants have been in use for years with successful tolerance in the human body.
Finally, Si3N4 is a highly versatile material. It has been used in sintered form to make polymers, bioactive glasses, and other composite ceramics and coatings that promote bone growth and retain its antibacterial properties.
Despite these obvious advantages, the study is limited by the use of powdered doped Si3N4 rather than the actual implant-grade material. Thus, further research is required to demonstrate that when used as part of a material or coating, such as paint, metal, fabric, or ceramic, it will continue to retain virucide activity.
For each application, the chemical process underlying the elution of ammonia and its conversion into virucidal compounds will need optimization. Adjustments may also be required to increase the rate of release of these compounds by altering the composition of this doped form of Si3N4, to enhance its effectiveness against viruses and bacteria and its safety to the host cells.
Finally, it is still unclear whether aqueous suspensions are truly necessary or simple physical contact with Si3N4 particles is enough to inactivate the virus – or both are required in unison.
The researchers sum up, “While Si3N4 is not suitable for ingestion or inhalation, its antiviral activity, which is not limited to SARS-CoV-2, suggests that it may be a fortuitous platform to engineer surfaces and personal protective equipment to discourage viral persistence, and thereby control the spread of COVID-19 and other diseases.”
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.