Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
Nitric Oxide in COVID-19
The severity of COVID-19 respiratory failure in some patients has taken the medical community by surprise. And in response, that community has been trying just about anything that seems reasonable in an effort to improve outcomes — and in some cases as last-resort measures to save lives.

Dr Lorenzo Berra
One of these is inhaled nitric oxide (NO). Medscape spoke with anesthesiologist Lorenzo Berra, MD, medical director of respiratory care at Massachusetts General Hospital and the Reginald Jenney Associate Professor of Anesthesia at Harvard Medical School, about his research, which aims to clarify the role of NO gas in the prevention and treatment of COVID-19. The interview has been edited for length and clarity.
What is NO? How is it used in the treatment of pulmonary disorders?
Inhaled NO gas is a selective pulmonary vasodilator (eg, it does not dilate the systemic circulation). It has primarily been used to increase systemic oxygenation by reducing the pulmonary vascular resistance in ventilated lung regions, thereby improving ventilation-perfusion (V/Q) matching. Nitric oxide is FDA-approved for use in premature and term neonates with persistent pulmonary hypertension, but it has also been used off-label for decades in the adult ICU and operating room to treat patients with pulmonary arterial hypertension, acute respiratory distress syndrome, hypoxia, and right ventricular failure after cardiac surgery or lung transplantation.
I understand that hundreds of hospitals are currently using NO as a rescue strategy for COVID-19. What evidence do we have to suggest that NO has therapeutic benefit in severe COVID-19?
Traditionally, NO has been used as a rescue therapy for patients who are extremely hypoxic and to prevent the need for extracorporeal membrane oxygenation or other aggressive treatment. But we have not had a recent large multicenter trial of NO since low tidal volumes have been widely used to to optimize lung ventilation in patients with ARDS. NO has been studied in small groups of ARDS patients — but not in a large randomized trial.
Everyone agrees that many patients with severe COVID-19 present with acute respiratory failure and hypoxemia. Ongoing studies are trying to better describe the phenotypes of respiratory failure in this disease. ARDS is a syndrome — with heterogeneous causes. Some patients develop the typical parenchymal disease, with V/Q mismatching and increased unperfused dead space.
COVID-19 produces an inflammatory process, with diffuse thrombosis in the pulmonary vascular bed. This inflammatory process is extremely important. A high degree of vascular involvement is often found on autopsy — much more than we expected.
Is NO expected to work in patients with different lung dynamics — low compliance and high compliance?
This is something we hope to learn from our research. From a strictly physiological point of view, NO should benefit both types of patients. For patients with evidence of low compliance, NO improves systemic oxygenation by improving V/Q mismatch. In high compliance, NO can also produce a significant improvement by reducing pulmonary artery pressure.
How do you assess the patient’s response to inhaled NO? Is it dose-dependent, and how long is it used?
NO has multiple effects in the pulmonary vasculature. Improved systemic oxygenation is usually seen within minutes, and it doesn’t require a high dose of NO. Some physicians start at 10-40 ppm. When we used it before COVID-19 in patients with acute respiratory failure, we would typically commence adding NO gas at 20 ppm or 40 ppm and wait for 1 hour. We seek at least a 20% improvement in oxygenation. That tells us whether the patient is a responder or a nonresponder to inhaled NO.
Patients can remain on this NO gas for weeks. Breathing high doses of NO inhibits the endogenous production of NO by NO synthase. Thus, it’s important that patients are slowly tapered off inhaled NO. If it is weaned off too quickly, there’s a risk for rebound pulmonary hypertension.
So, improved oxygenation is the acute response to NO among patients with COVID. But you are using NO differently, for the prevention of severe disease. What’s the basis for that?
In this disease, regardless of the oxygenation response, NO has the potential to be virucidal against the coronavirus responsible for COVID-19 disease. So we are not giving NO just for the improvement of oxygenation, which we usually observe, but also for this antiviral effect.
In addition to selective pulmonary vasodilation, NO has three other effects of interest in COVID19 therapy. The first is anti-inflammatory — NO has been shown to induce an anti-inflammatory response as well as a secondary antithrombotic effect, reducing the aggregation of platelets in lung vessels.
NO is also a bronchodilator. In our studies in the late 1990s, it was shown that adults with mild asthma had improved FEV1 results after inhaling NO.
But the most appealing potential effect of NO for COVID-19 that now needs confirmation is its antiviral action. Back in 2003–2004 during the SARS outbreak in China, a group of clinicians used NO in patients with SARS ARDS. Physicians reported that the chest X-rays of patients who had been given inhaled NO cleared much faster. Oxygenation improved, and the effect was lasting. In their report, the authors said they had never seen anything like that before with NO. Usually, there is an acute response of improved oxygenation, but not a rapid clearing of the chest x-ray. We began to question whether NO actually might have virucidal effects in COVID19.
Other groups tested the effects of NO donor drugs in patients infected with coronavirus species and found that the virus cleared faster and there was survival of eukaryotic cells. A similar effect has been shown in children with viral bronchiolitis, in whom a high dose (160 ppm) of NO gas led to clearing of the infection. A typical dose for children and adults in the ICU is from 5 ppm to 20 ppm. The maximum dose recommended by the FDA is 80 ppm — so we are talking about double or even higher doses of NO.
High dose NO is safe. For example, we have used a dose of 200 ppm successfully in a compassionate use protocol for a patient with cystic fibrosis who had multidrug-resistant bacterial pneumonia. The patient tolerated the use of high dose of NO well. Of note, the acute infection cleared, and we saw a change in the pattern of bacterial resistance.
Putting all this evidence together, we don’t have direct proof that NO will have antiviral effects in CoV-2, but because this coronavirus is 80% similar to CoV-1, we decided to test the hypothesis of whether a high dose of NO is virucidal.
If you are using NO primarily for its antiviral effects, do you start it at an earlier stage of illness than would be used as part of a rescue strategy for severe COVID-19?
Correct. The earlier we start inhaled NO treatment the better the result. We suspect that the optimum antiviral doses of NO are much higher than what we have conventionally used to improve V/Q matching or produce pulmonary vasodilation. That’s the basis for much of our ongoing research.
How does your research test the antiviral potential of inhaled NO in COVID-19?
Together with my teacher and mentor, Dr Warren Zapol, and my colleagues, Dr Fumito Ichinose, Dr Robert Kacmarek, Dr N. Stuart Harris, and Dr Ryan Carroll, we have four ongoing NO trials aimed at preventing COVID-19 symptoms or severity of disease in several patient groups, with more than 200 patients in each trial.
Severe COVID-19 study. The first is a classical multicenter randomized ICU trial in severely ill patients who are intubated for hypoxic respiratory failure and receiving mechanical ventilation. NO is started within 72 hours of admission and delivered continuously for 48 hours. We are looking for improvements in oxygenation, time spent on the ventilator, length of ICU stay, and length of hospital stay.

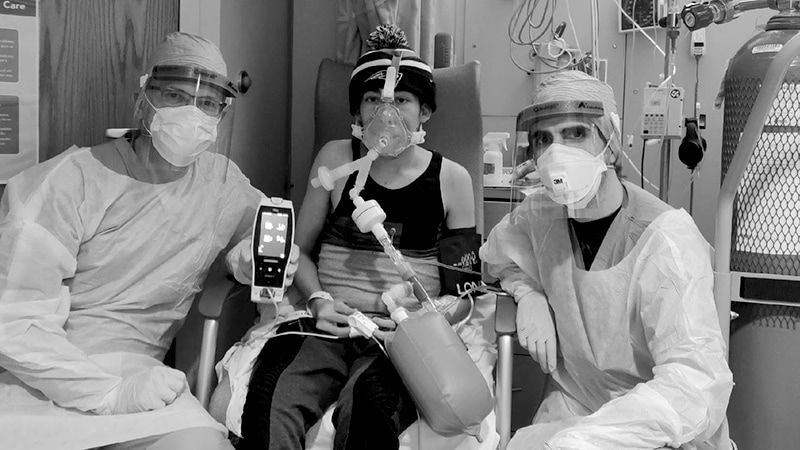
Patient (30-year old with COVID-19 symptoms) receiving NO inhalation treatment via the “magic flute” device. From L to R: Dr Lorenzo Berra, a patient, and Dr Bijan Safaee Fakhr.
Mild COVID-19 study. Because we believe the sooner you begin to administer NO, the better, the second randomized trial involves patients with COVID-19 who are hospitalized with pneumonia but are not intubated or receiving mechanical ventilation. The patient wears a tight-fitting mask that we developed and our own NO delivery device (called the “magic flute”) and the treatment group receives high dose NO inhalation therapy twice a day for 30 minutes until discharge or progression to intubation and ICU admission.
ED study. Dr N. Stuart Harris is the lead investigator of the third trial, which is a placebo-controlled, double-blind ED study. Just as early antibiotics can be lifesaving in sepsis, we believe this ED cohort (symptomatic, but without advanced pulmonary disease or injury) is most likely to respond well to this therapy. Patients with symptoms most likely related to COVID-19 are randomized, and the treatment group receives a 30-minute treatment of inhaled NO at doses up to 250 ppm. The patients are then discharged. Outcome measures include comparisons over the subsequent 28 days of need for return to the ED, hospitalization, intubation, and death.
Healthcare worker COVID-19 prevention study. The fourth trial uses NO to prevent symptoms in healthcare employees caring for COVID-19 patients. To date, more than 500 Massachusetts General Hospital employees have developed COVID-19. Volunteers randomized to the treatment group are given 10 minutes of NO inhalation at the beginning of each work shift and 10 minutes again at the end of their shift, breathing a dose of 160 ppm in air. Volunteers are not tested for the virus; the idea is to avoid symptoms and viral positivity at 28 days.
These are investigator-initiated trials, with the NO gas being provided by the manufacturer for the ICU and in-hospital trials. We are enrolling patients at a good pace.
We also have an in vitro study, in partnership with Dr Ron Corley of the National Emerging Infectious Diseases Laboratories, exploring the antiviral effects of NO donor compounds on the SARS-CoV-2 virus. Based on earlier work on SARS-CoV in 2003–2005, we are treating SARS-CoV-2 virus grown in cell culture with NO donors to determine the antiviral effect, as well as elucidate the mechanisms thereof.
Does NO have any systemic effects or potential adverse effects that must be monitored?
When NO gas mixes with oxygen as it is delivered to the patient, it forms nitrogen dioxide, which is toxic to lung tissue at over 2 ppm. Thus, we are monitoring nitrogen dioxide levels in all study patients.
NO gas is quickly metabolized by hemoglobin when it comes into contact with the lungs. It’s immediately converted to nitrate and nitrite, which are eventually cleared by the kidneys. Inhaled NO has no systemic hemodynamic effects about which we are aware.
There is a caveat in patients with a low ejection fraction, especially those with acute left heart failure and pulmonary edema. In these patients with high wedge pressures we avoid the use of NO.
Patients with methemoglobin reductase insufficiency can develop methemoglobinemia in the presence of NO, which needs to be treated immediately. It’s a very rare condition. We have treated thousands of patients with NO at MGH and I’ve never seen it. However, we monitor methemoglobin levels in all patients.
The other safety concern is renal injury. No randomized trials have demonstrated that NO causes renal injury, although there was one meta-analysis that concluded that NO in patients with ARDS and septic shock is associated with an increase in acute kidney injury. Many of these patients received NO as a last resort, and they were very sick when the gas was used. We don’t know whether the kidney injury is simply a confounding factor. We decided to include assessment of renal injury incidence and need for renal replacement therapy as a safety outcome.
Are you using NO for COVID-19 patients outside of the trial? And if a patient is in the control group and doesn’t receive NO or if patients progress in spite of NO for 48 hours, are they eligible for NO administration outside of the trial?
These are pragmatic trials. Thus, the ICU team is allowed to deliver NO gas (or any other clinically approved therapy) or use NO on a compassionate care basis in the control group.
Dr Lorenzo Berra is a staff anesthesiologist and intensivist at Massachusetts General Hospital and a member of Dr Warren Zapol’s MGH Anesthesia Center for Critical Care Research. His current patient care activities are focused on caring for critically ill patients and their families. His primary research and academic interests involve translational research to improve diagnosis, treatment, and care of critically ill patients with cardiopulmonary failure or severe infections.
Laura A. Stokowski, RN, MS, is the editor of Medscape Internal Medicine/Family Medicine, and has an intense interest in all things critical care.
For more news, follow Medscape on Facebook, Twitter, Instagram, and YouTube.