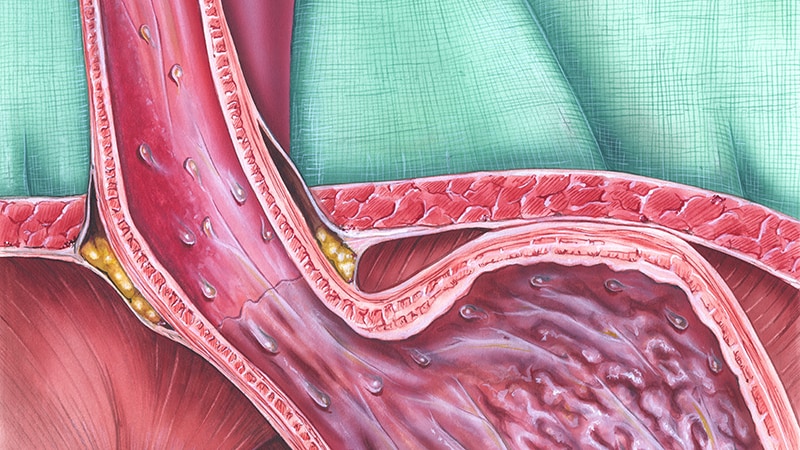
In patients with proton pump inhibitor (PPI)–dependent gastroesophageal reflux disease (GERD), a procedure known as endoscopic full-thickness plication (EFTP) — performed with the novel GERD-X device — improved both symptoms and quality of life, compared with a sham procedure. It also had few side effects and a short procedure time, according to a new randomized, controlled trial.
“It seems like it is a quick, easy-to-use procedure,” Gyanprakash A. Ketwaroo, MD, MSc, who is an assistant professor of medicine at Baylor College of Medicine, Houston, commented in an interview. Ketwaroo was not involved in the study.
“Even though the objective measures were not as good as perhaps you had hoped, the subjective outcomes were good. [And] it seems that it may have more of a long-term benefit, compared to some of the other endoscopic procedures. But that wasn’t a [primary] outcome of the study, and we still need more long-term studies to figure that out,” he added.
The research, led by Rakesh Kalapala, MD, DNB, and D. Nageshwar Reddy, MD, FACG, of the Asian Institute of Gastroenterology, Hyderabad, India, appeared in Gut.
The fact that the EFTP procedure is relatively simple could reduce cost and ease the learning curve, which in turn could broaden accessibility if more gastroenterologists are trained on it.
“There are not many gastroenterologists who offer endoscopic approaches to GERD therapy, so increasing that cohort [could] potentially have a huge impact given the number of patients who have GERD in this country, and especially given the rising and persistent concern over long-term use of PPIs,” said Ketwaroo.
Addressing the Drawbacks of Long-term PPI Use
Although PPIs are the most effective medical therapy for GERD, there are concerns that long-term use could increase the risk of acute and chronic kidney disease, hypomagnesaemia, Clostridioides difficile infection, and osteoporotic fractures. Surgical antireflux interventions are effective but may lead to dysphagia, bloating, and diarrhea.
EFTP applies transmural sutures to the gastroesophageal junction to strengthen the valvular mechanism, which reduces reflux. While the preponderance of published evidence supports the Esophyx device (EndoGastric Solutions), which has a 70% efficacy rate and few adverse events in one analysis, it requires advanced training and general anesthesia and takes 45-100 minutes.
“Endoscopic fundoplication is a minimally invasive antireflux therapy in patients with PPI dependence who refuse surgery; however, the majority of the endoscopic devices are cumbersome to use and robust data on their long-term efficacy are lacking,” Kalapala and colleagues noted in their new paper.
In 2014, the German company G-Surg introduced a novel endoscopic plication device called GERD-X. A prospective, single-arm study had shown efficacy in both patients taking PPIs and those with refractory GERD.
A Closer Look at the Device
To bolster that evidence, Kalapala and colleagues conducted this new single-center, randomized, sham-controlled trial with 70 enrollees with PPI-dependent GERD, of which 70% had nonerosive reflux disease (mean DeMeester score, 18.9). The median participant age was 36 years, and 71.4% were male. The average procedure time was 17.4 minutes.
Of the subjects in the treatment group, 65.7% achieved at least a 50% improvement in GERD health-related quality of life (GERD-HRQL) after 3 months, compared with 2.9% in the sham group (P < .001). The median percentage improvement in GERD-HRQL score was higher in the treatment group at 6 months (81.4% vs. 8.0%; P < .001) and at 12 months (92.3% vs. 9.1%; P < .001). Similar improvements were seen at 6 months and 12 months in heartburn symptom score (75.0% vs. 13.0% and 89.7% vs. 15.4%, respectively; P < .001 for both) and regurgitation symptom score (96.2% vs. 6.9% and 100% vs. 3.4%, respectively; P < .001 for both). At 12 months, 62.8% of the treatment group no longer took PPIs, compared with 11.4% of the sham group (P < .001).
Objective measures of improvement were more modest. The treatment arm trended toward a reduction in esophageal acid exposure from baseline at 3 and 12 months, but the difference was not statistically significant. The median percentage of time with esophageal pH below 4 and the DeMeester score were similar between the groups at 3 and 12 months. The researchers also noted trends toward fewer reflux events in 24 hours in the treatment group at 6 months (P = .072) and 12 months (P = .051).
The treatment group had fewer non–acid reflux episodes at 12 months versus baseline (P = .038), but there was no difference in the median number of acid reflux episodes in 24 hours.
“Our study found endoscopic full-thickness fundoplication, using a novel device, was safe and significantly improved GERD-related quality of life and severity of reflux symptoms at short and long terms, compared with a sham procedure,” wrote the authors.
“This endoluminal procedure with a short operating time and very few side effects is a promising alternative option to surgery in appropriately selected group of patients, who may not want to continue PPI long term,” they concluded.
The authors of the study disclosed no external funding. Ketwaroo has no relevant financial disclosures, although he is on the editorial advisory board for GI & Hepatology News.
This article was updated May 6, 2021.
This article originally appeared on MDedge.com, part of the Medscape Professional Network.