Studies have shown that mortality rates in hospitalized patients with COVID-19 are high, ranging between 10% and 30%. REGEN-COV®, a combination of monoclonal antibodies casirivimab and imdevimab, is approved in the United States and other regions for emergency treatment of COVID-19 outpatients with mild to moderate SARS-CoV-2 infection and post-exposure prophylaxis.
REGEN-COV has been shown to reduce viral load, hospitalization, mechanical ventilation, all-cause mortality, and decrease symptom duration in COVID-19 patients. In addition, data show that a single subcutaneous dose of REGEN-COV is highly effective for preventing both symptomatic and asymptomatic SARS-CoV-2 infection, thus lowering the risk of developing COVID-19 by approximately 80%.
Previously, a UK-based open-label trial RECOVERY reported that REGEN-COV improved overall survival in patients with a poor immune response at baseline and decreased duration of hospitalization.
Researchers from Regeneron Pharmaceuticals, Inc., Brown University, NYC Health + Hospitals/Lincoln, and the Oregon Clinic, have now reported the results from the first phase 1, 2, & 3 double-blinded, placebo-controlled trial to evaluate the safety, efficacy, and tolerability of REGEN-COV in hospitalized COVID-19 patients on low-flow or no supplemental oxygen. This study is currently available on the medRxiv* preprint server.
Study design
The trial was conducted at 103 sites in the United States, Chile, Brazil, Moldova, Mexico and Romania, and included 1,364 adult patients on low-flow or no supplemental oxygen. The patients were characterized at baseline for viral load and SARS-CoV-2 endogenous immune response and randomized to receive a single intravenous dose of 2.4 g REGEN-COV (1.2 g casirivimab and 1.2 g imdevimab), 8.0 g REGEN-COV (4.0 g casirivimab and 4.0 g imdevimab), and placebo. The phase 2 trial included patients requiring no supplemental oxygen, and phase 3 had patients requiring low-flow oxygen.
Out of the 1,364 patients with low-flow or no supplemental oxygen, 1,336 were treated and 1,197 (nearly 90%) tested positive for SARS-CoV-2, with 406 from the 2.4 g REGEN-COV group, 398 from the 8.0 g REGEN-COV group, and 393 from the placebo group. Efficacy was analyzed by a modified full analysis set (mFAS) which excluded patients who were SARS-CoV-2 negative at baseline.
REGEN-COV reduced viral burden and improved all-cause mortality in the overall population
The results showed that REGEN-COV effectively reduced viral load in patients on low or no supplemental oxygen and in the overall population, with higher reductions in seronegative patients. Similar results were found with both the doses of REGEN-COV. REGEN-COV also improved all-cause mortality in seronegative, high viral load, and overall populations at day 29 and through day 57, with similar results observed in relation to hospital discharge and readmission.
Overall, REGEN-COV treatment resulted in a reduction of viral load, mechanical ventilation requirement, and death in hospitalized patients with COVID-19 on low-flow or no supplemental oxygen and in the overall population with more benefit in seronegative patients.
Adverse events
Patients in the placebo group experienced more adverse events than patients in the REGEN-COV group on low-flow oxygen (27.9% in placebo vs. 23.8% in REGEN-COV) and no supplemental oxygen (21.7% in placebo vs. 15.3% in REGEN-COV). Treatment-emergent adverse events were also comparatively high in the placebo group than the REGEN-COV group on low-flow oxygen (15.4% in placebo vs. 11.5% in REGEN-COV) and no supplemental oxygen (7.6% in placebo vs. 3.8% in REGEN-COV). These findings confirmed the treatment benefit of REGEN-COV.
Findings support REGEN-COV use in hospitalized COVID-19 patients, regardless of serostatus
In conclusion, REGEN-COV is a strong antiviral monoclonal antibody treatment that reduces viral burden and risk of death or mechanical ventilation in COVID-19 patients with low-flow or no supplemental oxygen, with greater efficacy in seronegative patients and decreased all-cause mortality in the overall population.
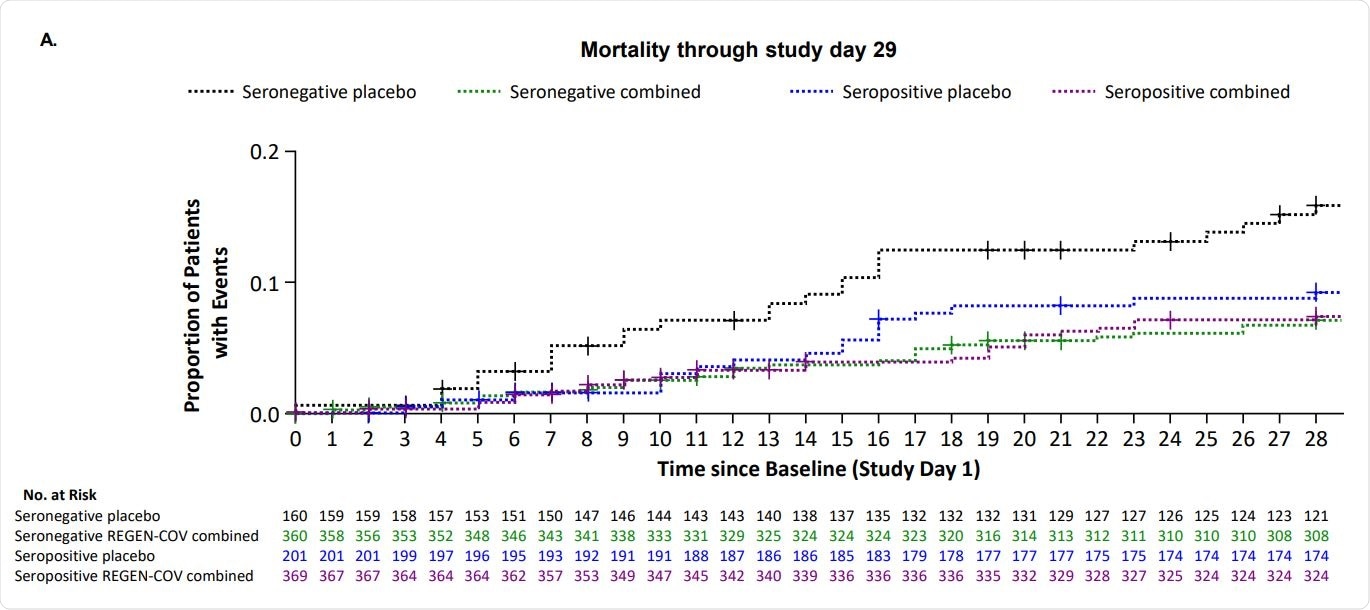
Overall, no safety concerns were noted with REGEN-COV in seronegative or seropositive patients. There were fewer deaths through day 29 in seropositive patients given REGEN-COV compared to those given placebo.
The findings of this study support the use of REGEN-COV in hospitalized COVID-19 patients, regardless of their serostatus. More studies are needed to analyze further the potential clinical benefit of REGEN-COV in seropositive patients.
“REGEN-COV is the first monoclonal antibody therapy, and the first SARS-CoV-2 antiviral, that significantly lowers the viral load and reduces mortality in hospitalized patients with Covid-19.”
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- REGEN-COV for the Treatment of Hospitalized Patients with Covid-19 Selin Somersan-Karakaya, Eleftherios Mylonakis, Vidya P. Menon, Jason C. Wells, Shazia Ali, Sumathi Sivapalasingam, Yiping Sun, Rafia Bhore, Jingning Mei, Jutta Miller, Lisa Cupelli, Andrea T. Hooper, Jennifer D. Hamilton, Cynthia Pan, Viet Pham, Yuming Zhao, Romana Hosain, Adnan Mahmood, John D. Davis, Kenneth C. Turner, Yunji Kim, Amanda Cook, Bari Kowal, Yuhwen Soo, A. Thomas DiCioccio, Gregory P. Geba, Neil Stahl, Leah Lipsich, Ned Braunstein, Gary A. Herman, George D. Yancopoulos, David M. Weinreich, the Covid-19 Phase 2/3 Hospitalized Trial Team, medRxiv, 2021.11.05.21265656; doi: https://doi.org/10.1101/2021.11.05.21265656, https://www.medrxiv.org/content/10.1101/2021.11.05.21265656v2
.jpg)