In a recent report posted to the medRxiv* preprint server, researchers determined immune responses generated by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron BA.2 and BA.1 sublineage infections in prior virus-infected and naïve subjects.
Study: Omicron BA.1 and BA.2 immune response in naïve and prior infected persons. Image Credit: Ninc Vienna / Shutterstock
Background
The significantly transmissible SARS-CoV-2 B.1.1.529 (Omicron) variant has supplanted the prior viral variants and is less vulnerable to neutralizing antibodies induced by coronavirus disease 2019 (COVID-19) or vaccination. Therefore, the SARS-CoV-2 Omicron BA.2 sublineage is currently dominant.
Studies have reported that COVID-19 vaccinated subjects infected with the SARS-CoV-2 Omicron BA.1 sublineage have generated detectable neutralizing antibody levels against BA.2 and BA.1 sublineages. However, the capacity of BA.2 infection to elicit neutralizing antibodies in COVID-19 vaccine-naïve individuals, either with or without prior SARS-CoV-2 infection, is yet to be determined.
About the study
In the current study, the investigators quantified the neutralizing antibody responses and antibody concentrations in the blood samples of 74 outpatients infected with the SARS-CoV-2 Omicron BA.2 or BA.1 sublineages between December 2021 and March 2022 in Australia. The subjects submitted written informed consent before enrolling in the study. Further, the data of the study volunteers used in this work were anonymized and coded. This research exposed participants to the least amount of risk possible.
Although none of the study volunteers were COVID-19 vaccinated, 26 participants had a history of SARS-CoV-2 infection with a previous variant. The authors measured the humoral responses of the study volunteers between one and three weeks after the documented Omicron BA.2 or BA.1 infections. The scientists used antibody concentrations of the individuals infected with the SARS-CoV-2 Beta variant for comparison.
End-point binding immunoglobulin G (IgG) antibody titers to several antigens derived from SARS-CoV-2 were quantified using the Meso Scale Discovery (MSD) technology. The V-plex multi-spot SARS-CoV-2 serology kits were used to assay the 1) SARS-CoV-2 nucleocapsid (N), spike (S), and 2) SARS-CoV-2 Beta, Alpha, Delta, Omicron, and Gamma S subdomains.
Further, the scientists quantified the neutralization ability of the samples by performing the angiotensin-converting enzyme 2 (ACE2) binding inhibition analysis. The V-PLEX SARS-CoV-2 ACE2 neutralization kit was used to quantify antibodies that hinder the binding of ACE2 with the S subdomains of the SARS-CoV-2 variants.
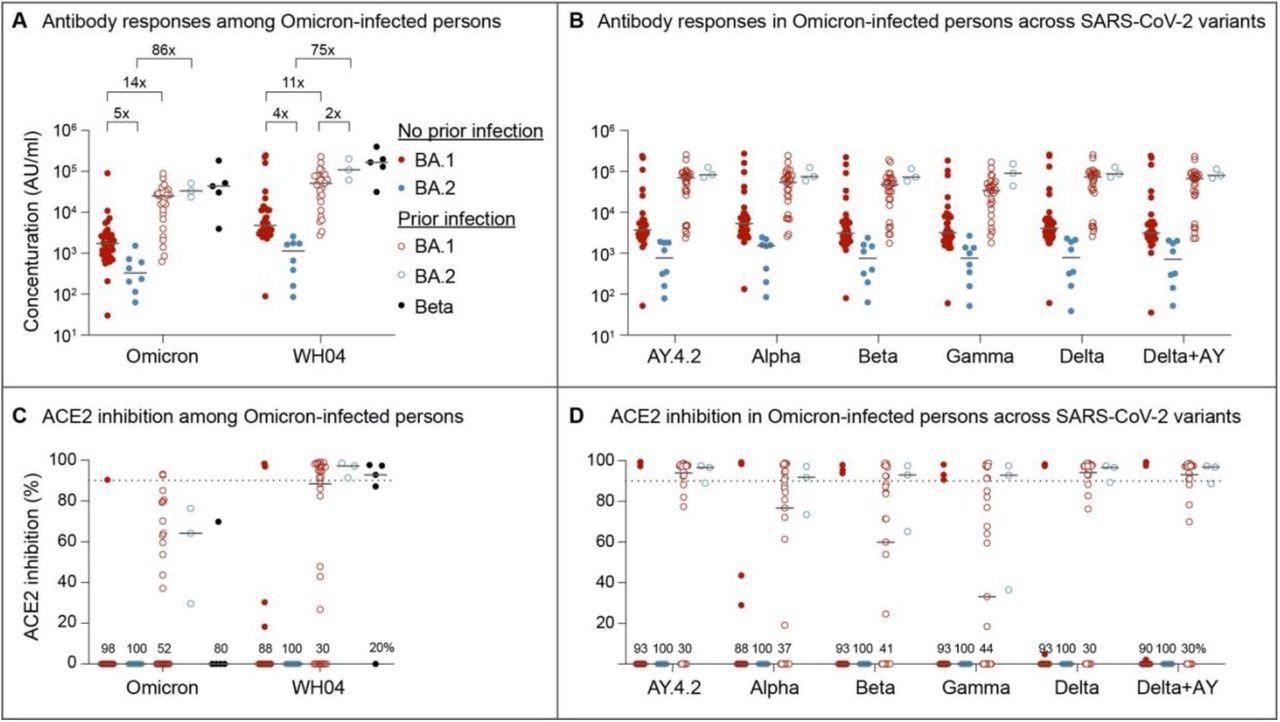
Serology and Neutralizing antibody responses in outpatients infected with the omicron BA.1 and BA.2 sublineages. Panel A and B show plasma IgG antibody binding the SARS-CoV-2 RBD (spike) from the ancestral and Omicron strains (A) as well as other variants (B) in the unvaccinated BA.1 and BA.2 Omicron patients without or with prior SARS-CoV-2 infection. Panel C and D show neutralizing antibody response by measuring inhibition of binding between ACE2 and SARS-CoV-2 spike protein. Antibody titers were measured 1-3 weeks after the infection. Results are shown as the median and dots for each data (No prior infection, BA.1, n = 40; No prior infection, BA.2, n = 10; Prior infection, BA.1, n = 23; Prior infection, BA.2, n = 3; Beta infection, n = 4).
Results and discussions
The study results demonstrated that over 20% of the SARS-CoV-2 Omicron BA.1 infected subjects had severe or moderate COVID-19, while this was not observed in BA.2 infected subjects. In the SARS-CoV-2 infected and vaccination-naïve individuals, both anti-SARS-CoV-2 ancestral and Omicron S IgG concentrations were four to five times reduced after BA.2 infection relative to BA.1. On the contrary, antibody levels in COVID-19 convalescent subjects were 11 to 89-times higher, with no variation in concentrations between the BA.1 and BA.2 infected subjects.
The antibody titers from Beta-infected subjects were similar to the COVID-19 convalescents who experienced Omicron BA.2 or BA.1 infections. Across all SARS-COV-2 variants studied, the differential response in antibody production following BA.2 and BA.1 infection was constant.
Neutralizing capacities against the SARS-CoV-2 Omicron and ancestral strains were comparably reduced in the previously uninfected and unvaccinated subjects infected with either the Omicron BA.2 or BA.1 sublineages. Identical to the overall antibody concentrations, neutralizing activity was heightened in the subjects with COVID-19 history following BA.2 or BA.1 infections. In addition, neutralizing activity in SARS-CoV-2 Beta-infected and COVID-19 convalescent BA.2- or BA.1-infected subjects was comparable, and it was consistent among all SARS-CoV-2 variants assessed.
On the whole, the present study showed that the immune response after the SARS-CoV-2 Omicron BA.2 infections in the SARS-CoV-2 antigen-naïve subjects was much lower than the BA.1 infections. The authors proposed that the diminished neutralization response after BA.2 infections might promote extended circulation of BA.2 in the community due to reduced immunity against SARS-CoV-2 re-infections. The scientists further stated that the COVID-19 vaccination per current standards after the resolution of Omicron infections might prevent this threat.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.