In a recent study under consideration at Scientific Reports and posted to the Research Square* preprint server, researchers evaluated the impact of time on the sensitivity of four severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) serological assays using samples from the coronavirus disease 2019 (COVID-19) staff testing of antibody responses study (Co-Stars) in the United Kingdom (UK).
Following the emergence of SARS-CoV-2 in late 2019, a wide range of serological tests has been developed to estimate the seroprevalence of SARS-CoV-2, eligibility for COVID-19 booster vaccinations, and anti-SARS-CoV-2 antibody therapies. The regulatory authorities approved the SARS-CoV-2 serological assays after evaluating their performances two to three weeks after infection on reference sera samples, including SARS-CoV-2-negative or positive controls.
Further, the SARS-CoV-2 serological analyses are essential to understand the immune correlates of protection from future COVID-19 waves following natural infection or vaccination. Nonetheless, there is only limited knowledge about the performance of these serological tests over time following SARS-CoV-2 infection.
Study: The Influence of Time on the Sensitivity of SARS-CoV-2 Serological Testing. Image Credit: Andrei Dubadzel / Shutterstock.com
About the study
In the study, the researchers concurrently compared the results of the multiplexed spike (S), nucleoprotein (N) and receptor-binding domain (RBD) Meso Scale Discovery (MSD) assay, the Roche-S assay, the Roche-N assay, and the Abbott-N assay on monthly samples from healthcare workers (HCWs) for 250 days following SARS-CoV-2 infection procured in connection with Co-Stars.
Initially, the sera samples from 3657 subjects were screened using enzyme-linked immunosorbent assay (ELISA), and then samples were collected monthly from those HCWs who were SARS-CoV-2-positive up to approximately six months.
The team evaluated the proportion of samples that remained SARS-CoV-2 seropositive as time passed by survival analysis. They further estimated the decay rate of the SARS-CoV-2 S antibody and the N antibody for the four diagnostic assays employing a previously published mathematical model fitted to the current data.
Study findings
The results show that nearly 98% of the subjects were SARS-CoV-2-seropositive by two or more assays evaluated. The quantitative Roche-S and MSD assays indicated that the SARS-CoV-2 S antibody production remained high for about six months after the infection. However, all N antibody assays showed the deterioration of the SARS-CoV-2 N antibodies with time. The waning effect of the N antibody was slower in the Roche-N assay than in the Abbott-N assays.
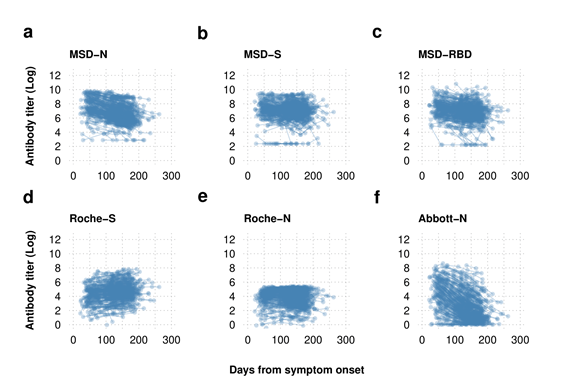
Log transformed serial serological antibody titer data plotted by time from symptom onset Antibody dynamics are dependent on the assay used with the sensitive Roche-S and MSD-S assay demonstrating maintenance of the spike protein antibody while the nucleoprotein antibody is shown to wane with the MSD and Abbott-N assays but to a lesser extent with the Roche-N assay.
Every assay evaluated in the current study demonstrated significant sensitivity following 50 days of SARS-CoV-2 infection. While almost half of the Abbott-N assays exhibited a SARS-CoV-2-negative result following 175 days of COVID-19, the results of the semi-quantitative Roche-N assay and the MSD-N assay remained seropositive. Further, the Roche-S and the MSD-S assays also maintained the SARS-CoV-2 seropositivity up to 200 days following the infection.
The MSD-RBD assay inferred some evidence for reductions of SARS-CoV-2-seropositivity with time. However, the top-performing quantitative Roche-S assay indicated no proof of the waning of S antibody titers and even a slight increase after 200 days of symptom onset of the SARS-CoV-2 infection.
Of 183 SARS-CoV-2-seropositive samples from the quantitative MSD-N assay with an antibody titer value less than 403, 137 were seronegative by the Abbott-N assays. Hence, suggesting the Abbott-N assays were not suitable for seroprevalence investigations. The deterioration in the performance of the Abbott-N assays was associated with the decline of detectable antibody titers over time.
Conclusions
According to the researchers, this is the first study evaluating the sensitivity of various COVID-19 diagnostic tests using longitudinally procured serological samples from SARS-CoV-2-infected patients in parallel. The study findings indicate that although several SARS-CoV-2 serological analyses demonstrated high sensitivity during the initial three weeks following the infection, it was not the case six months after COVID-19.
In detail, the study showed that the Abbott-N assay failed to detect the SARS-CoV-2 antibodies over time following the infection. By contrast, the MSD and the Roche assays demonstrated high sensitivity up to 200 days following SARS-CoV-2 infection. This suggests that the sensitivity of SARS-CoV-2 serological testing varies depending on the assay technique used.
Collectively, the study highlights the significance of caution while using the Abbott assay in SARS-CoV-2 seroprevalence investigations and clinical decision-making, such as determining the eligibility for anti-SARS-CoV-2 antibody therapy or COVID-19 booster vaccination due to its reducing SARS-CoV-2 sensitivity as time elapses.
Moreover, the presence of the neutralizing S antibodies is linked to lower chances of SARS-CoV-2 re-infection and severe form of the disease. Therefore, the long-lasting detectable SARS-CoV-2 neutralizing S antibody titers by the quantitative Roche-S assay adds on the proof for the prolonged protection against the pre-existing strains of SARS-CoV-2 after the natural infection.
Furthermore, the study indicates the applicability of the Roche-S assay in diagnosing multisystem inflammatory syndrome in children (MIS-C), long coronavirus disease (COVID), and SARS-CoV-2 following vaccination.
*Important notice
Preprints with Research Square publish preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
