The novel investigational peptide inhibitor zilucoplan (UCB) continues to show promise in the treatment of adults with generalized myasthenia gravis (gMG).
Top-line results from the phase 3 RAISE trial showed the drug met the primary and all key secondary endpoints and was well tolerated, UCB has announced.
Zilucoplan is a self-administered, subcutaneous peptide inhibitor of complement component 5 (C5) that binds to and inhibits C5 activation, which prevents the formation of the membrane attack complex.
Compared with placebo, zilucoplan led to “clinically meaningful and statistically significant” improvement from baseline in Myasthenia Gravis-Activities of Daily Living Profile total score at week 12, which was the primary outcome measure, the company reported.
All key secondary endpoints were also met, including significant improvements from baseline in Quantitative Myasthenia Gravis score, Myasthenia Gravis Composite score, and MG-QoL15r score at week 12.
In addition, zilucoplan was well-tolerated, with no major unexpected safety findings identified relative to earlier studies of the drug. Serious treatment-emergent adverse events were similar in the zilucoplan and placebo groups.
Detailed results from the RAISE trial will be presented at a medical meeting this year, the company said.
“Important Step Forward”
The phase 3 results of zilucoplan in gMG support earlier positive phase 2 data, which were reported at the time by Medscape Medical News.
gMG is a rare and chronic disease affecting almost 200,000 patients in the United States, the European Union, and Japan.
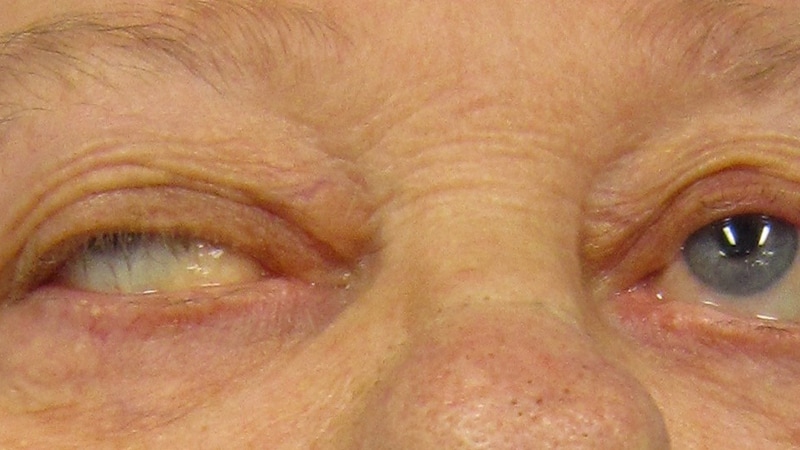
Patients living with gMG can suffer a variety of symptoms, including drooping eyelids, double vision, and difficulty swallowing, chewing, and talking.
They can also experience severe life-threatening weakness of the respiratory muscles. About 80% of patients with the condition progress to generalized muscle weakness.
“gMG patients can experience varying and debilitating symptoms that impact their everyday lives in unique ways,” lead investigator James F. Howard, Jr, MD, professor of neuromuscular disease at the University of North Carolina, Chapel Hill, said in a company news release.
“These exciting results give us an additional reason to believe that zilucoplan can offer an important step forward in addressing the unmet needs of people living with gMG,” Howard said.
Based on the RAISE study findings, UCB said it plans to progress with regulatory filings for zilucoplan in gMG in the United States, European Union, and Japan beginning later this year.
For more Medscape Neurology news, join us on Facebook and Twitter.